The Fascinating Chemistry Behind The H3O Reaction
The H3O reaction is a captivating chemical phenomenon that plays a crucial role in various scientific fields, particularly in acid-base chemistry. Understanding this reaction not only aids in grasping fundamental chemical concepts but also has significant implications in industrial applications and environmental science. In essence, the H3O reaction involves the hydronium ion (H3O+), which is formed when water molecules interact with acids, leading to a range of reactions that can influence everything from pH levels in solutions to biochemical processes in living organisms.
When we talk about the H3O reaction, we delve into the world of acids, bases, and their interactions. The hydronium ion itself is an essential player in these interactions, representing the transfer of protons in aqueous solutions. This process is fundamental to understanding how acids behave in water and how they can affect other substances. The H3O reaction is not just a theoretical concept; it has real-world applications that can be observed in everyday life, from the taste of our food to the functioning of our bodies.
Furthermore, the significance of the H3O reaction extends beyond traditional chemistry. It also intersects with environmental science, biology, and even industrial processes. By comprehending the mechanisms behind the H3O reaction, scientists and researchers can develop better methods for pollution control, improve agricultural practices, and even innovate in the field of pharmaceuticals. As we explore the depths of this reaction, we uncover a wealth of knowledge that can contribute to advancements in science and technology.
Read also:The Ultimate Guide To Finding The Best Asian Food Near Me
What is the H3O Reaction?
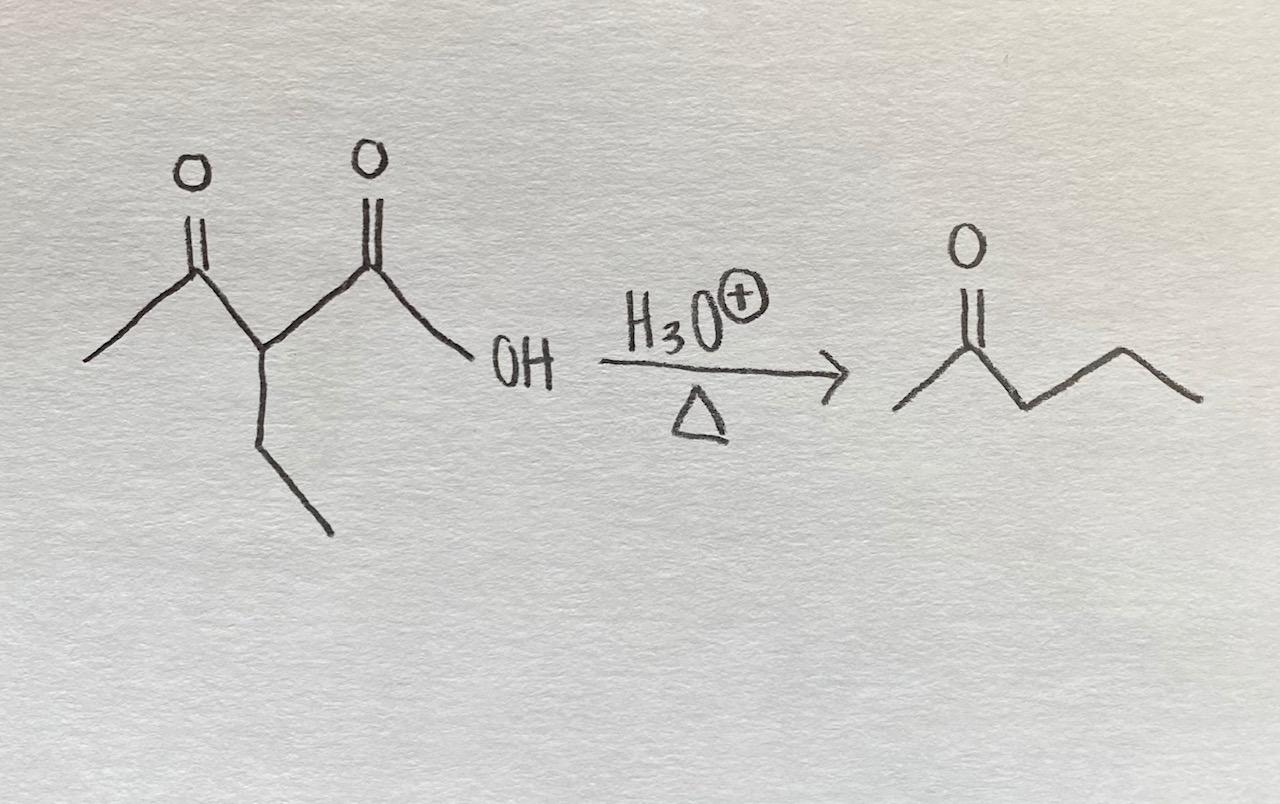
The H3O reaction, also known as the hydronium ion formation, occurs when an acid donates a proton (H+) to a water molecule (H2O). This process leads to the creation of the hydronium ion (H3O+), which is pivotal in understanding acid-base chemistry. The general equation for this reaction can be represented as:
HA + H2O ⇌ H3O+ + A-
In this equation, HA represents the acid, and A- represents the conjugate base formed after the acid donates the proton.
How Does the H3O Reaction Impact pH Levels?
The H3O reaction directly influences the pH levels of a solution. The pH scale measures the concentration of hydronium ions in a solution; therefore, a higher concentration of H3O+ results in a lower pH, indicating a more acidic solution. Conversely, a lower concentration of hydronium ions corresponds to a higher pH, which signifies a basic or alkaline solution. Understanding this relationship is crucial for various applications, including:
- Water quality assessment
- Soil chemistry in agriculture
- Biological processes in organisms
What Role Does the H3O Reaction Play in Biological Systems?
In biological systems, the H3O reaction is vital for numerous biochemical processes. For instance, enzymes often require specific pH levels to function optimally, which is directly influenced by the concentration of hydronium ions. Additionally, the H3O reaction is crucial in cellular respiration and photosynthesis, where the balance of acids and bases must be maintained for proper functioning. The involvement of the H3O reaction in these processes highlights its importance in sustaining life.
How is the H3O Reaction Applied in Industry?
The industrial applications of the H3O reaction are diverse and impactful. Industries such as pharmaceuticals, agriculture, and food processing leverage the principles of acid-base chemistry to optimize their processes. For example:
Read also:The Comprehensive Guide To Understanding T65533rban A Look Into Its Cultural Historical And Social Significance
- In pharmaceuticals, the pH of solutions can affect drug solubility and stability.
- In agriculture, managing soil pH through the H3O reaction can enhance nutrient availability to plants.
- In food processing, controlling acidity can influence flavor, preservation, and safety.
What Are Some Common Misconceptions About the H3O Reaction?
Despite its significance, several misconceptions about the H3O reaction exist. Here are a few common ones:
- Some believe that hydronium ions are the only form of protons in solution, overlooking the role of free protons.
- There is a misconception that all acids produce the same concentration of H3O ions, which varies based on the strength of the acid.
- Many people think the H3O reaction is only relevant in chemistry labs, ignoring its daily implications.
How is the H3O Reaction Studied in the Laboratory?
In laboratory settings, the H3O reaction is studied using various techniques, including:
- pH measurements with pH meters or indicators to determine the concentration of H3O ions.
- Titration methods to analyze acid-base reactions and calculate the concentration of hydronium ions.
- Spectroscopy techniques to observe changes in molecular structures during the reaction.
Conclusion: Why Understanding the H3O Reaction Matters?
In conclusion, the H3O reaction is a fundamental concept in chemistry with far-reaching implications in various fields. By comprehending this reaction, we gain insights into the behavior of acids and bases, the functioning of biological systems, and the optimization of industrial processes. The H3O reaction is not merely a theoretical concept; it is a vital component of our world, influencing everything from our health to the environment. As we continue to research and explore this fascinating area of chemistry, the potential for innovation and improvement in our lives remains vast.
Article Recommendations